The raw materials related to our business
MOLYBDENUM TRIOXIDE
CAS Registry Number: 1313-27-5
Structure: MoO3
Molecular Formula: MoO3
1. Description
Molybdenum trioxide occurs as an odorless powder, or in granular or crystalline form. It is white, or slightly yellow to slightly bluish, depending on the temperatures. Molybdenum trioxide is sparingly soluble in water, very soluble in excess alkali (with formation of molybdates), and soluble in a concentrated mixture of nitric and sulfuric acids. Molybdenum trioxide readily combines with acids and bases to form a series of polymeric compounds.
2. Physical Properties
| Molecular Weight | 143.94 |
| Boiling Point | 1,155°C |
| Melting Point | 795°C |
| Density/Specific Gravity | 4.696 at 26/4°C |
3. Application
Make molybdenum powder, petroleum catalyst, pigment, alloy raw materials, etc.
4. Specification
| Chemical elements contents (%) | |||||||
|---|---|---|---|---|---|---|---|
| Not less than | Not more than | ||||||
| Mo | Pb | S | P | C | Cu | Sb | Sn |
| 57.00 | 0.05 | 0.10 | 0.05 | 0.10 | 0.30 | 0.04 | 0.04 |
5. Size
0-4mm 90% min
6. Packing
Package One: 25KG
Package Two: 250KG
Package Three: And also we have customize package as your requirement
7. Marking
Commodity name, gross weight, net weight and company name
8. Origin
Viet Nam
Molybdenum
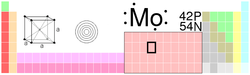
Molybdenum (pronounced /məˈlɪbdənəm/, from the Greek meaning "lead-like"), is a Group 6 chemical element with the symbol Mo and atomic number 42. It has the sixth-highest melting point of any element, and for this reason it is often used in high-strength steel alloys. Molybdenum is found in trace amounts in plants and animals, although excess molybdenum can be toxic in some animals. Molybdenum was discovered in 1778 by Carl Wilhelm Scheele and first isolated in 1781 by Peter Jacob Hjelm.
Characteristics
Molybdenum is a transition metal with an electronegativity of 1.8 on the Pauling scale and an atomic mass of 95.9 g/mole.It does not react with oxygen or water at room temperature. At elevated temperatures, molybdenum trioxide is formed in the reaction 2Mo + 3O2 → 2MoO3.
In its pure metal form, molybdenum is silvery white with a Mohs hardness of 5.5, though it is somewhat more ductile than tungsten. It has a melting point of 2623°C, and only tantalum, osmium, rhenium, and tungsten have higher melting points.Molybdenum burns only at temperatures above 600°C. It also has the lowest heating expansion of any commercially used metal.
Applications
The ability of molybdenum to withstand extreme temperatures without significantly expanding or softening makes it useful in applications that involve intense heat, including the manufacture of aircraft parts, electrical contacts, industrial motors, and filaments.Molybdenum is also used in alloys for its high corrosion resistance and weldability.Most high-strength steel alloys are .25% to 8% molybdenum.Despite being used in such small portions, more than 43 million kg of molybdenum is used as an alloying agent each year in stainless steels, tool steels, cast irons, and high-temperature superalloys.
Because of its lower density and more stable price, molybdenum is implemented in the place of tungsten. Molybdenum can be implemented both as an alloying agent and as a flame-resistant coating for other metals. Although its melting point is 2623 °C, molybdenum rapidly oxidizes at temperatures above 760°C, making it better-suited for use in vacuum environments. Molybdenum 99 is used as a parent radioisotope to the radioisotope Technetium 99, which is used in many medical procedures
Molybdenum disulfide (MoS2) is used as a lubricant and an agent. It forms strong films on metallic surfaces, and is highly resistant to both extreme temperatures and high pressure, and for this reason, it is a common additive to engine motor oil; in case of a catastrophic failure, the thin layer of molybdenum prevents metal-on-metal contact. Lead molybdate co-precipitated with lead chromate and lead sulfate is a bright-orange pigment used with ceramics and plastics.Molybdenum trioxide (MoO3) is used as an adhesive between enamels and metals. Molybdenum powder is used as a fertilizer for some plants, such as cauliflower.
Also used in NO, NO2, NOx analyzers in power plants for pollution controls. At 350 °C the element acts as a catalyst for NO2/NOx to form only NO molecules for consistent readings by infrared light.
Vanadium
Vanadium (IPA: /vəˈneɪdiəm/) is a chemical element that has the symbol V and atomic number 23. A soft and ductile element, vanadium naturally occurs in about 65 different minerals and is used mainly to produce certain alloys. It is one of the 26 elements found in most living organisms.
Characteristics
Vanadium is a soft and ductile, silver-grey metal. It has good resistance to corrosion by alkalis, sulfuric and hydrochloric acid. It oxidizes readily at about 933 K (660 C). Vanadium has good structural strength and a low fission neutron cross section, making it useful in nuclear applications. Although a metal, it shares with chromium and manganese the property of having valency oxides with acid properties.
Common oxidation states of vanadium include +2, +3, +4 and +5. A popular experiment with ammonium vanadate NH4VO3, reducing the compound with zinc metal, can demonstrate colorimetrically all four of these vanadium oxidation states. An oxidation state of +1 is rarely seen.
Applications
Approximately 80% of vanadium produced is used as ferrovanadium or as a steel additive. Other uses:
- In such alloys as
- specialty stainless steel, e.g. for use in surgical instruments and tools.
- rust resistant and high speed tool steels.
- mixed with aluminium in titanium alloys used in jet engines and high-speed airframes.
- Vanadium steel alloys are used in axles, crankshafts, gears, and other critical components.
- It is an important carbide stabilizer in making steels.
- Because of its low fission neutron cross section, vanadium has nuclear applications.
- Vanadium foil is used in cladding titanium to steel.
- Vanadium-gallium tape is used in superconducting magnets (175,000 gauss).
- Vanadium pentoxide V2O5 is used as a catalyst in manufacturing sulfuric acid (via the contact process) and maleic anhydride. It is also used in making ceramics and glass manufacturing.
- Glass coated with vanadium dioxide VO2 can block infrared radiation (and not visible light) at a specific temperature.
- Electrical fuel cells and storage batteries such as vanadium redox batteries.
- Added to corundum to make simulated alexandrite jewelry.
- Vanadate electrochemical conversion coatings for protecting steel against rust and corrosion.
- Lithium vanadium oxide is proposed for use as a high energy density anode for lithium ion batteries, at 745Wh/l when paired with a lithium cobalt oxide cathode.[2]
- Used to make lacrosse shafts.
- Possibly used to make Wootz steel and Damascus steel.
Nickel
Nickel (pronounced /ˈnɪkəl/) is a metallic chemical element with the symbol Ni and atomic number 28.
Characteristics
Nickel is a silvery-white metal that takes on a high polish. It belongs to the transition metals, and is hard and ductile. It occurs most usually in combination with sulfur and iron in pentlandite, with sulfur in millerite, with arsenic in the mineral nickeline, and with arsenic and sulfur in nickel glance.
Similar to the massive forms of chromium, aluminium and titanium, nickel is a very reactive element, but is slow to react in air at normal temperatures and pressures. Due to its permanence in air and its inertness to oxidation, it is used in coins, for plating iron, brass, etc., for chemical apparatus, and in certain alloys, such as German silver.
Nickel is magnetic, and is very often accompanied by cobalt, both being found in meteoric iron. It is chiefly valuable for the alloys it forms, especially many superalloys, and particularly stainless steel. Nickel is also a naturally magnetostrictive material, meaning that in the presence of a magnetic field, the material undergoes a small change in length. In the case of Nickel, this change in length is negative (contraction of the material), which is known as negative magnetostriction.
The most common oxidation state of nickel is +2, though 0, +1, +3 and +4 Ni complexes are observed. It is also thought that a +6 oxidation state may exist, however, results are inconclusive.
The unit cell of nickel is a face centered cube with a lattice parameter of 0.352 nm giving a radius of the atom of 0.125 nm.
Nickel-62 is the most stable nuclide of all the existing elements; it is more stable even than Iron-56.
Applications
Nickel is used in many industrial and consumer products, including stainless steel, magnets, coinage, and special alloys. It is also used for plating and as a green tint in glass. Nickel is pre-eminently an alloy metal, and its chief use is in the nickel steels and nickel cast irons, of which there are innumerable varieties. It is also widely used for many other alloys, such as nickel brasses and bronzes, and alloys with copper, chromium, aluminium, lead, cobalt, silver, and gold.
Nickel consumption can be summarized as: nickel steels (60%), nickel-copper alloys and nickel silver (14%), malleable nickel, nickel clad, Inconel and other Superalloys (9%), plating (6%), nickel cast irons (3%), heat and electric resistance alloys, such as Nichrome (3%), nickel brasses and bronzes (2%), others (3%).
In the laboratory, nickel is frequently used as a catalyst for hydrogenation, most often using Raney nickel, a finely divided form of the metal.
Nickel has also been often used in coins, or occasionally as a substitute for decorative silver. The American 'nickel' five-cent coin is 75% copper. The Canadian nickel minted at various periods between 1922-81 was 99.9% nickel, and was magnetic.
Nickel(III) oxide is used as the cathode in many rechargeable batteries, including nickel-cadmium, nickel-iron and nickel-metal hydride batteries.
Cobalt
Cobalt (pronounced /ˈkoʊbɒlt/) is a hard, lustrous, silver-grey metal, a chemical element with symbol Co. It is found in various ores, and is used in the preparation of magnetic, wear-resistant, and high-strength alloys. Its compounds are used in the production of inks, paints, and varnishes.
Characteristics
Cobalt is a silver or gray ferromagnetic metal. Pure cobalt is not found in nature, but compounds of cobalt occur naturally in many forms. Small amounts of it are found in most rocks, soil, water, plants, and animals. It is the element of atomic number 27. The Curie temperature is 1388 K with 1.6~1.7 Bohr magnetons per atom. In nature, it is frequently associated with nickel, and both are characteristic ingredients of meteoric iron. Mammals require small amounts of cobalt which is the basis of vitamin B12. Cobalt-60, an artificially produced radioactive isotope of cobalt, is an important radioactive tracer and cancer-treatment agent. Cobalt has a relative permeability two thirds that of iron. Metallic cobalt commonly presents a mixture of two crystallographic structures hcp and fcc with a transition temperature hcp→fcc of 722 K. Cobalt has a hardness of 5.5 on the Mohs scale of mineral hardness.
Applications
- Alloys, such as
- Superalloys, for parts in gas turbine aircraft engines.
- Corrosion- and wear-resistant alloys.
- High speed steels.
- Cemented carbides (also called hard metals) and diamond tools.
- Magnets and magnetic recording media
- Alnico magnets.
- Samarium-cobalt magnets.
- Catalysts for the petroleum and chemical industries, e.g. for hydroformylation and oxidation.
- Electroplating because of its appearance, hardness, and resistance to oxidation.
- Drying agents for paints, varnishes, and inks.
- Ground coats for porcelain enamels.
- Pigments (cobalt blue and cobalt green)
- Cobalt blue glass
- Lithium ion battery electrodes.
- Steel-belted radial tires.
- Purification of histidine-tagged fusion proteins in biotechnology applications.
Tungsten
Tungsten (pronounced /ˈtʌŋstən/), also known as wolfram (/ˈwʊlfrəm/), is a chemical element that has the symbol W and atomic number 74.
Characteristics
A steel-gray metal, tungsten is found in several ores, including wolframite and scheelite. It is remarkable for its robust physical properties, especially the fact that it has the highest melting point of all the non-alloyed metals and the second highest of all the elements after carbon. Tungsten is often brittle and hard to work in its raw state; however, if pure, it can be cut with a hacksaw.The pure form is used mainly in electrical applications, but its many compounds and alloys are used in many applications, most notably in light bulb filaments, X-ray tubes (as both the filament and target), and superalloys. Tungsten is also the only metal from the third transition series that is known to occur in biomolecules.
Applications
Because of its ability to produce hardness at high temperatures and its high melting point (the second highest of any known element), elemental tungsten is used in many high-temperature applications. These include light bulb, cathode-ray tube, and vacuum tube filaments, as well as heating elements and nozzles on rocket engines. The high melting point also makes tungsten suitable for aerospace and high temperature uses which include electrical, heating, and welding applications, notably in the gas tungsten arc welding process (also called TIG welding).
Due to its conductive properties, as well as its relative chemical inertia, tungsten is also used in electrodes, and in the emitter tips of field emission electron-beam instruments, such as focused ion beam (FIB) and electron microscopes. In electronics, tungsten is used as an interconnect material in integrated circuits, between the silicon dioxide dielectric material and the transistors. Additionally, it is used in the manufacture of metallic films, which replace the wiring used in conventional electronics with a coat of tungsten (or molybdenum) on silicon.[14]
Tungsten chemical compounds are used in catalysts, inorganic pigments (e.g. tungsten oxides), and also as high-temperature lubricants (tungsten disulfide). Tungsten carbide (WC) is used to make wear-resistant abrasives and cutters and knives for drills, circular saws, milling and turning tools used by the metalworking, woodworking, mining, petroleum and construction industries.Tungsten oxides are used in ceramic glazes and calcium/magnesium tungstates are used widely in fluorescent lighting. Crystal tungstates are used as scintillation detectors in nuclear physics and nuclear medicine. Other salts that contain tungsten are used in the chemical and tanning industries.